Регистрация и верификация в Pin-Up.ru: как быстро создать аккаунт
В букмекерской конторе Pin-Up.ru легко начать игру: регистрация занимает минуту, а если у вас есть подтвержденный Qiwi-кошелек, идентификация пройдет автоматически.
Все о букмекере
Чтобы делать ставки на спорт в букмекерской конторе Pin-Up.ru, нужно пройти регистрацию и идентификацию. Без идентифицированного аккаунта не получится пополнить счет и разместить ставку.
Для регистрации используйте только свои настоящие данные , это правило любой легальной букмекерской конторы. Если регистрироваться на чужой номер телефона или использовать подложные документы, букмекер заблокирует аккаунт.
После регистрации подтвердите возраст. Перед пополнением счета необходимо пройти идентификацию – подтвердить, что вам уже исполнилось 18 лет. В некоторых случаях идентификация проходит автоматически, подробнее расскажем дальше.
Регистрируйтесь только на официальном сайте. Недобросовестные компании используют бренды других букмекерских контор, чтобы зарабатывать на чужом имени. Чтобы не переводить деньги мошенникам, проверьте, что вы создаете аккаунт именно на официальном сайте pin-up.ru, или просто нажмите на кнопку ниже.
Как создать аккаунт
Пройти регистрацию можно на официальном сайте букмекера pin-up.ru или в мобильном приложении. На апрель 2021 года приложение есть только для смартфонов и планшетов на Android. Чтобы скачать и установить приложение, зайдите на сайт с мобильного устройства, затем откройте меню и нажмите на «Установить Андроид».
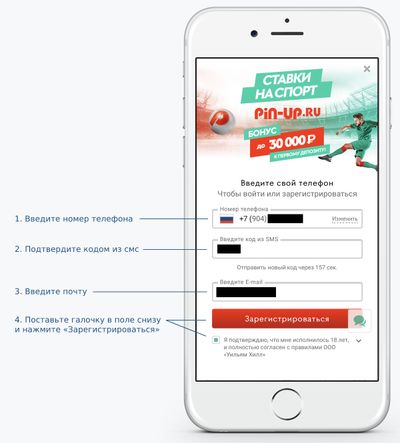
В верхней части сайта или приложения вы увидите две кнопки: «Войти» и «Регистрация». Нажмите на любую – откроется форма регистрации.
Чтобы создать аккаунт, укажите номер телефона и подтвердите его кодом, который придет в смс-сообщении. Затем чуть ниже введите почту, подтвердите согласие с правилами и нажмите «Зарегистрироваться».
Если вы правильно ввели код из смс-сообщения, регистрация на Pin-Up.ru будет завершена
После регистрации на почту придет письмо с подтверждением, что аккаунт создан. В этом же письме букмекер попросит вас подтвердить почту: для этого просто перейдите по ссылке, которую вы увидите чуть ниже. Подтверждать почту необязательно: можно делать ставки и без этого.
Аккаунт готов, но впереди еще один шаг: подтвердить личные данные. Эта процедура называется верификацией или идентификацией.
Как пройти идентификацию
В Pin-Up.ru есть два вида идентификации: начальная и полная. Проще пройти начальную идентификацию, но в этом случае максимальные суммы на пополнение счета и снятие выигрыша будут ограничены. С полной идентификацией ограничений нет.
| Начальная идентификация | Полная идентификация | |
|---|---|---|
| Как пройти | Ввести паспортные данные | Прийти с паспортом в офис Qiwi, «Мегафон», Contact или «Связной» |
| Ограничение на операции в сутки | 60 000 рублей | Нет ограничений |
| Ограничение на операции в месяц | 200 000 рублей | Нет ограничений |
Чтобы идентифицировать аккаунт на сайте Pin-Up.ru, перейдите в личный кабинет, нажмите «Лимиты» и выберите один из способов подтверждения возраста.
Способ 1: ввод паспортных данных. Введите паспортные данные в форму и нажмите «Сохранить». Букмекер проверит данные и откроет доступ к пополнению счета. Проверка занимает 10–15 минут.
Способ 2: фотографии паспорта. Возьмите паспорт и сделайте несколько фотографий:
- основной разворот паспорта;
- страница с регистрацией (пропиской);
- селфи с паспортом в руках.
Загрузите фотографии в форму и подождите, пока букмекер проверит данные. На фотографиях должно быть хорошо видно каждую букву, иначе придется делать фото заново. Проверка занимает 10–15 минут, если фотографии получились с первого раза.
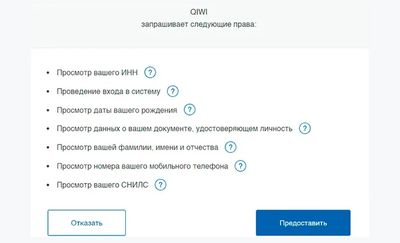
Способ 3: через Госуслуги. Если у вас есть подтвержденная учетная запись на портале Госуслуги, предоставьте к ней доступ. Идентификация пройдет моментально. Если учетной записи нет, пройдите регистрацию на Госуслугах, а затем приходите с паспортом в МФЦ и подтвердите личные данные.
При идентификации через Госуслуги вы автоматически передаете букмекеру все необходимые персональные данные
Pin-Up.ru работает с ЦУПИС Qiwi – через эту организацию проходят все расчеты между игроком и букмекером. Для вас это означает две вещи:
- Когда вы завершаете идентификацию в Pin-Up.ru, то автоматически создаете себе подтвержденный Qiwi-кошелек. Поэтому при регистрации в других БК, которые работают с ЦУПИС Qiwi, верифицировать аккаунт не придется.
- Если у вас уже есть подтвержденный Qiwi-кошелек, то идентификация в Pin-Up.ru пройдет автоматически – сможете пополнять счет и ставить на спорт сразу после создания аккаунта.
Поэтому если вы планируете играть в других букмекерских конторах, которые обслуживает ЦУПИС Qiwi, удобнее создать кошелек на официальном сайте Qiwi и пройти проверку. Это можно сделать онлайн, понадобится только паспорт. Как именно пройти идентификацию через Qiwi – читайте в нашей статье про Qiwi ЦУПИС.
Когда букмекер проверит документы, на главной странице сайта и мобильного приложения появится такое уведомление
Вопросы и ответы
Почему при регистрации не приходит смс с кодом?
Смс-сообщение приходит через 5–10 секунд на тот номер, который вы указали при регистрации. Если ошиблись с телефоном, нажмите «Изменить номер» в форме регистрации или пройдите регистрацию заново. Если не помогло, обратитесь в службу поддержки Pin-Up.ru: 8 (800) 222-77-88.
Почему не пришло письмо с подтверждением регистрации аккаунта?
Есть два варианта: вы ошиблись при вводе электронного адреса во время регистрации или письмо попало в папку «Спам». Если вы уверены, что правильно ввели адрес, а в «Спаме» ничего нет, позвоните в поддержку или не обращайте на это внимания, потому что делать ставки можно и без подтверждения почты.
Как пройти идентификацию, если у меня нет гражданства РФ?
Достаточно идентифицировать Qiwi-кошелек. Для этого приходите в офис Qiwi, Contact, «Мегафон» или «Связной» с нотариально заверенным переводом паспорта и любым документом, который подтверждает право нахождения на территории России: миграционная карта, разрешение на временное проживание или вид на жительство.
Что делать, если после идентификации у меня изменился телефон или паспортные данные?
Напишите в поддержку и расскажите, какие именно данные у вас изменились. Затем отправьте фотографии нового паспорта или договора с мобильным оператором на почтовый адрес, который узнаете от сотрудника БК.
Если я не помню пароль, можно ли зарегистрировать новый аккаунт?
Нет, аккаунт может быть только один. Если вы забыли пароль, попробуйте восстановить его через форму входа на сайт, или напишите в поддержку. За регистрацию двух аккаунтов букмекер заблокирует оба.
Также игрок можете делать прямом в режиме Live.Более продвинутые юзеры, могут объединить несколько пари в одну, тем самым сделав экспресс. Это поможет увеличить выигрыш, если, конечно, все ставки сыграют.
Утилита позволяет составлять прогнозы на разные виды спорта в live формате или прематче. Для удобной навигации предстоящие игры рассортированы по категориям. Среди них: футбол, баскетбол, теннис, волейбол, хоккей и другие.
В каждом матче существуют сотни возможных исходов, на которые можно заключать пари как в LIVE, так и до начала матча.
пользователей
Languages of the mobile betting app
Pin-up,ставки на спорт, спорт, Pinup, ставка, спортивные ставки, Pin up, прогнозы, ставки на футбол, ѕрогнозы на спорт, Pin up
Добрый день, благодарим за ваш отзыв. Будем и дальше делать всё, чтобы вы наслаждались игрой вместе с нами!)
Букмекерская контора Pin-Up.ru опубликовала условия акции, в рамках которой клиенты компании могут каждый понедельник получать фрибеты за правильные ответы на квиз.
Condividi su email
Читайте другие статьи, похожие на Пинап Рабочее Зеркало На Сегодняшний ищут:
- Лига Ставок Pin Up Вин
- Pinup Ставки
- Букмекера Пин Ап
- Пин Ап Бесплатная Ставка
- Pin Up Как Сделать Ставку Вин
- Онлайн Ставки В Pin Up
- Ставка Pin Up Официальный